
Quality assurance
Innovative products that meet global quality standards
Since its inception , United Orthopaedic Corporation has been committed to promoting quality, value and innovative product design. QA teams, consisting of engineers, scientists and QA/QC inspectors, continuously monitor operations to ensure that they comply with strict international manufacturing and performance standards. To date, United Orthopaedic Corporation has more than 70 granted or pending patents for its high-value arthroplasty products and devices.


ISO-compliant quality management systems
United Orthopaedic Corporation uses Quality Management Systems (QMS) in accordance with the requirements of ISO 13485: 2016, as determined by the BSI. We also hold CE markings for our European operations as well as approvals from FDA 510(k) (United States), TFDA (Taiwan), NMPA (China), KFDA (Korea), PMDA (Japan), ANVISA (Brazil) and other distributed markets.
“Clinically proven design”
United Orthopaedic Corporation draws on direct practitioner input, clinical research and extensive market research when designing its hip and knee replacement implants and devices. To ensure consistent quality, reliability and efficiency, we execute and manage all critical manufacturing processes in-house:
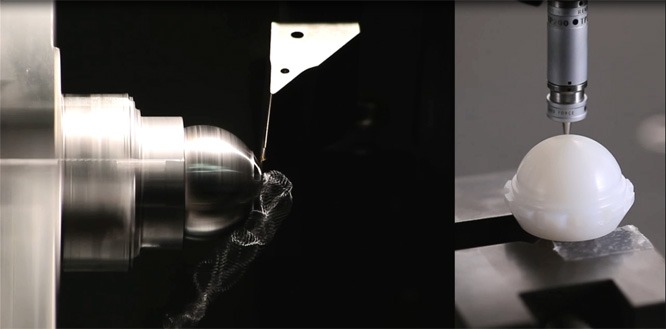
- Forming: forging and precision casting
- Cutting: milling, turning, threading, grinding and filing
- Coating: blasting, tumbling, polishing, sintering
- Heat treatment: annealing, solution treatments
- Cleaning, chemical treatment: passivation, electrode polishing, anodising
- Inspection: measuring instruments, optical comparator, coordinate measuring machine, roundness, roughness, hardness
Do you prefer
By Phone?
You can reach us by phone from Monday to Friday,
from 08:30 to 12:30 and from 13:30 to 17:30
About us

United Orthopedic France is the French subsidiary of the international United group, set up in July 2016 in Nancy.
Contact
United Orthopedic Corporation
7, Allée des Peupliers
54180 Houdemont
France